Beijing Scinovo Laboratories Co., Ltd. (referred to as Scinovo Laboratories) was established in November 2016 and is affiliated to Sunnovo Pharm (successfully listed on the STAR Market of the Shanghai Stock Exchange on June 21, 2021, stock code 688621). In Jinweikai Biological Science and Technology Park in Advanced Business Park, Fengtai District, Beijing, an innovative drug PK/PD research platform with an area of more than 2000 square meters and in line with GCP and GLP standards has been built. The company has a registered capital of 20 million yuan. At present, the total investment exceeds 120 million yuan. It can provide comprehensive PK/PD research services and bioanalysis services meeting GLP standards, including pharmacokinetic bioanalysis services, bioanalysis, biomarker bioanalysis and pharmacodynamics services, central laboratory services, pathology services and pharmacometrics Studies.


1) Laboratory construction in compliance with international standards
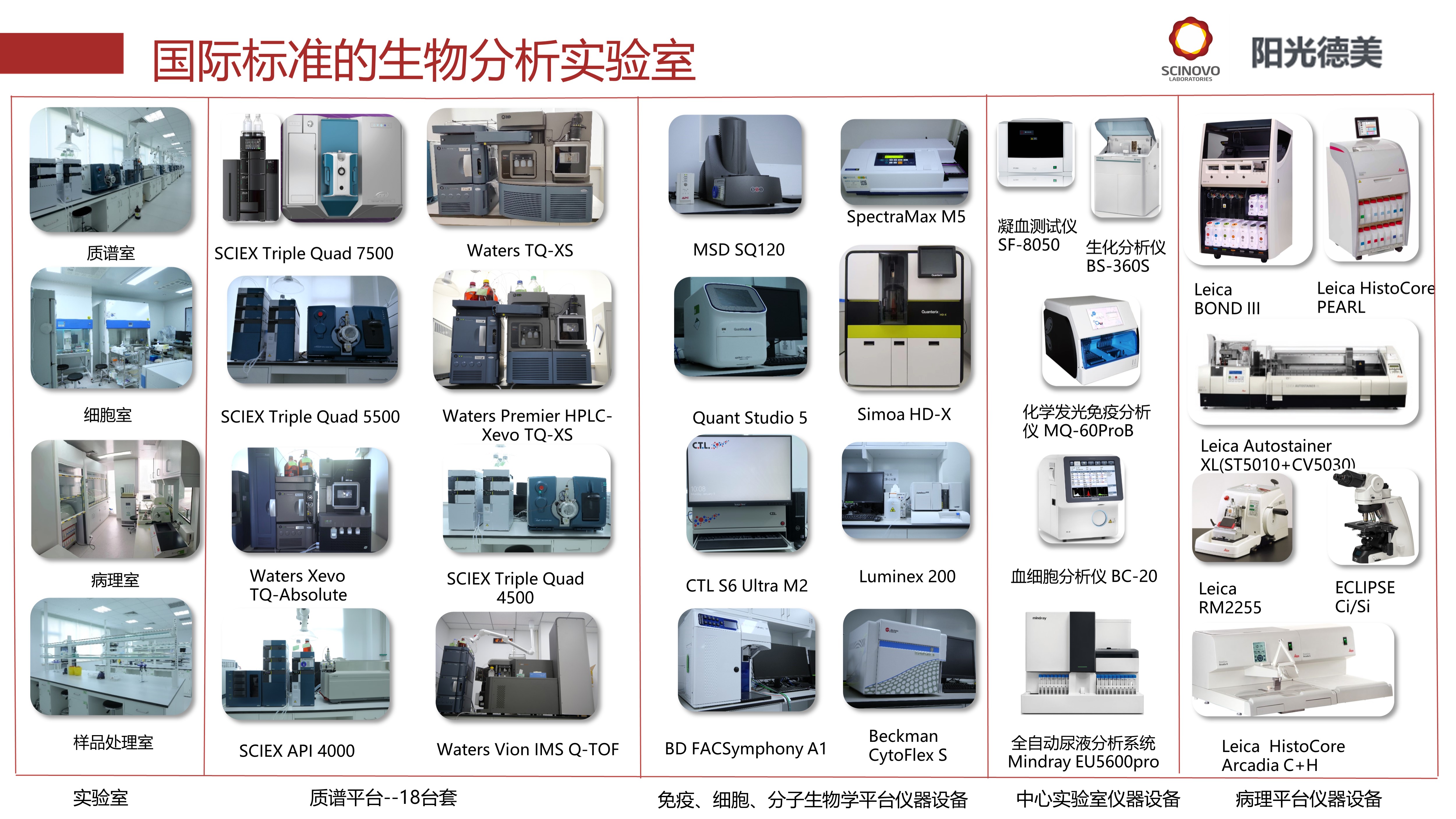
Scinovo Laboratories has a bioanalysis laboratory, a P2-level cell laboratory and a PCR laboratory that meet GLP and GCP requirements. Relying on the technical advantages of mass spectrometry analysis, immunoassay, cell biology, molecular biology, and pathology platforms, as well as the service advantage of a one-stop central laboratory covering from sample collection kit preparation to sample detection completion, it can provide comprehensive PK/PD research services from tissue level, cell level, protein level to molecular level and bioanalysis services that meet GLP standards.

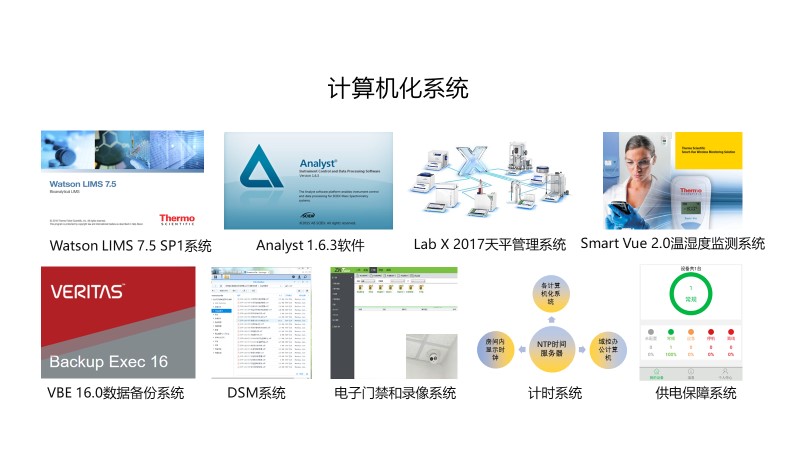
2) Safe and efficient computerized management system and IT system
Scinovo Laboratories is equipped with a scientific and efficient computerized intelligent management system to meet international industry standards. Using this system, sample and testing lifecycle management can be controlled easily .
3) Strong technical capabilities and precise service capabilities
Scinovo Laboratories has accumulated experience in the development and verification of nearly 100 kinds of drugs, including: small molecule drugs, peptide and protein drugs, synthetic isotope drugs, biomarkers, etc.. The advantages of the project are: development and compound of endogenous drug detection methods Development of drug detection methods, and simultaneous detection of multiple metabolites. At the same time, Scinovo also provides customized services and precise services according to customer needs to help the project successfully declare.
4) Perfect quality management system
The quality system of Scinovo is based on the requirements of China, the United States and the European Union, and can meets the requirements of drug license applicantion both China and international. Scinovo passed ISO17025 laboratory certification and obtained a certificate in February 2019. Quality assurance department works independently to inspect and supervise the research projects, institutional facilities, and key processes according to the work plan, ensuring that all research work meets the requirements of the lab's internal quality management system and regulations, and thus all test data is accurate and reliable.









